Definitive Closure.
Definitive Closure.
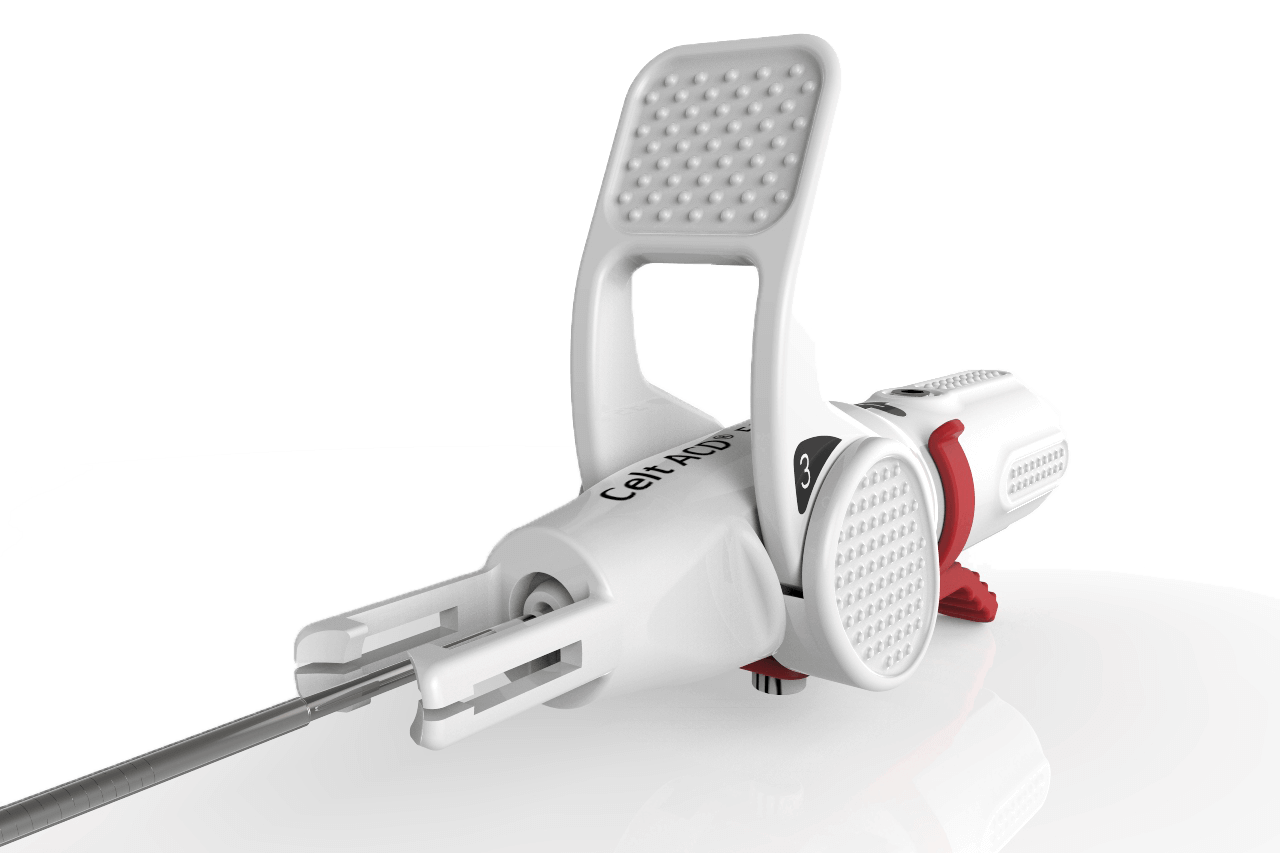
The CELT ACD System is a next generation vascular closure device providing safe, effective and reliable closure of femoral arterial access sites following catheterization procedures with 5F to 7F sheaths.1 2 3
Designed for procedural efficiency, the CELT ACD device provides rapid and complete hemostasis in anticoagulated patients without limitations in calcified vessels.1 2 3
The one-piece implant actively seals the puncture to minimize risk of late bleeding.3
This safe and effective approach to small bore arterial closure enables early ambulation and discharge, per physician discretion, to simplify workflow, transform the patient experience, and reduce overall cost of care. 3 4 5 6
REFERENCES
1Wong et al. Catheterisation and Cardiovascular Interventions; 90:756-765 (2017)
2Cahill et al. Heart; 100: A44 (2014)
3ICELT IFU (Rev 3)
4Hussain. Presentation at Charing Cross, UK. April 2019
5Economic model, Company data on file.
6Jost et al. Letter to the editor. 09.02.20. Journal of Vascular Access.
Active, sustained hemostasis
-
Effective closure with a mean time to hemostasis (TTH) of < 1 minute11,2
- 99.3% procedural success in clinical studies1,2
- No limitations in calcified vessels3
- 0% late access site-related bleeding at X timepoint (following discharge)1,2
Simple & Reliable Deployment
- Uses existing procedural sheath, does not require a sheath exchange1
- Minimal training required
- One-piece implant design conforms to arteriotomy and provides secure closure in a wide variety of anatomy, including calcified vessels
- Small implant footprint facilitates reaccess1
Improved procedural experience
- 99.3% freedom from major complication (including vascular repair, retroperitoneal bleeding, bleeding requiring transfusion or access site complications)
-
Rapid hemostasis enables prompt ambulation1,2
- Clinical use in 400 patients demonstrates a mean time to ambulation of 17 minutes (range: 5 – 25 min)2
- Eliminates the need for extended bedrest in a flat position or monitoring for late bleeds
Elective closure of a femoral arterial puncture inter-operatively to facilitate immediate ambulation for patient to have a bowel movement, then return to the procedure room for re-access, case completion and re-closure through the same common femoral artery.
Objectives: This study compared the performance of Celt ACDVR, a novel stainless steel based vascular closure device versus manual compression for femoral arteriotomy site hemostasis in patients undergoing percutaneous coronary procedures.
Conclusions: After 6-F percutaneous invasive procedures in fully anticoagulated patients, TTH was signifi-cantly reduced in patients assigned to Celt ACDVR compared to patients managed with MC. The 30-day rates of vascular complications were similarly low in both groups. (CELT ACD Trial; NCT01600482)
BGF announces multi-million investment in Vasorum – high-growth medical device business based in Dublin Ireland
BGF – Ireland’s largest growth capital investor – has completed a €6 million equity investment in Vasorum Limited. Vasorum is a Dublin based high-growth medical device business specialising in sophisticated devices used to close arterial punctures in vascular, cardiology, radiology and neuro-radiology procedures. BGF invests in minority positions in growing businesses and aims to support and…